TruScreen Group Limited (NZX/ASX:TRU) is pleased to announce the preliminary publication, on 25 July 2024, of a study titled “Beyond Tradition: Investigating TruScreen’s Performance Versus Pap Smear in Cervical Cancer Detection” on Research Square1 Link. The preliminary publication is subject to peer review.
Highlights
- 507 women tested from 2021-2022, results published in July 2024
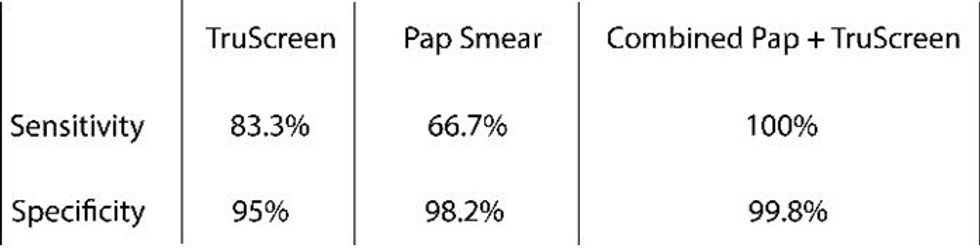
- High Sensitivity and Specificity: TruScreen demonstrated a sensitivity of 83.3% and a specificity of 95% for detecting cervical lesions (neoplasms), compared to 66.7% and 98.2% for Pap smear, respectively1.
- Real-Time Results: The TruScreen device provides immediate screening results, eliminating the need for laboratory equipment and pathology staff.
- Practical and Reliable: The study confirmed that TruScreen is a practical and reliable screening tool, suitable for use in various healthcare settings.
The authors concluded that TruScreen “represents a reliable, practical screening tool for cervical neoplasms” and that their results “provide an evidence-based approach for policymakers when selecting the optimal cervical cancer screening strategy in countries without an established national screening program.”
This study reinforces TruScreen’s commitment to providing innovative and accessible healthcare solutions. The positive results validate TruScreen’s technology and opens new opportunities in the global healthcare market.
For more information, please visit our website at www.truscreen.com.
Click here for the full ASX Release
This article includes content from Truscreen, licensed for the purpose of publishing on Investing News Australia. This article does not constitute financial product advice. It is your responsibility to perform proper due diligence before acting upon any information provided here. Please refer to our full disclaimer here.
Credit: Source link




