Investor Insight
BlinkLab’s transformative AI-based healthcare technology is at the forefront of innovations in the global medical field that are quickly gaining traction among keen investors.
Overview
BlinkLab (ASX:BB1) offers a smartphone-based diagnostic platform that leverages computer vision, artificial intelligence (AI) and machine learning (ML). A company started by neuroscientists at Princeton University, Blinklab has developed its novel technology over several years, providing an app-enabled, smartphone-based diagnostic tool for evaluating children with neurodevelopmental conditions such as autism and ADHD.
The app turns a smartphone into a diagnostic tool that helps to conduct remote and rapid tests that can assist in diagnosing neurodevelopmental conditions. BlinkLab’s smartphone app provides a screening tool that can help with diagnoses much earlier than the age that children are typically assessed at present (approximately 5-6 years old). It is also a remote (i.e., accessible) and inexpensive means of beginning the assessment process, which can typically be very costly and take up to multiple years currently.
BlinkLab’s smartphone app facilitates early diagnosis, reduces costs, and improves accuracy.
BlinkLab’s smartphone-based technology, which uses AI and machine learning (ML), makes it attractive to investors. Like other industries, AI is becoming very popular in the healthcare sector. According to Statista, the AI healthcare market is expected to proliferate from $11 billion in 2021 to $187 billion in 2030. The increasing use of AI is driven by advanced ML algorithms, access to data, and use of 5G technology. AI and ML technologies can evaluate and analyze enormous volumes of data faster than humans.
Artificial intelligence, and particularly machine learning, has the potential to serve as the great equaliser for many behavioural healthcare concerns like autism. According to recent data, 97 percent of adults in the United States own a cellular device, and nine in ten own a smartphone. A 2022 Global State of Digital report by We Are Social shows 66.9 percent, or about 5.34 billion, of the world’s population are mobile users. As these percentages continue to rise and internet-powered devices become ubiquitous, access to digital health services can become democratised on a global scale. While autism spectrum disorder (ASD) services are currently restricted to relatively privileged populations, digital solutions powered by emerging data, science, and methodologies can make access to autism therapy more accessible.
Large players are investing in this segment to tap into the vast potential of these new technologies. One such example was Pfizer’s acquisition of ResApp. In October 2022, Pfizer acquired Queensland University startup ResApp Health for $179 million. ResApp developed a smartphone technology to detect respiratory diseases using cough analysis accurately.
Furthermore, big tech companies such as Apple, Amazon, Microsoft and Alphabet are also now venturing into the AI healthcare market.

Company Highlights
- Australia-based BlinkLab (ASX:BB1) is focused on transforming mental healthcare through an AI-enabled smartphone application, a breakthrough technology developed with Princeton University.
- The company’s innovative approach leverages the power of smartphones, AI and machine learning to deliver screening tests specifically designed for children as young as 18 months old. This marks a significant advancement, considering traditional diagnoses typically occur around five years of age, often missing the crucial early window for effective intervention.
- Once approved by regulators, this cutting-edge digital technology is poised to capture the imagination of both investors and major pharmaceutical companies, eager to embrace transformative solutions in healthcare.
- BlinkLab is led by an experienced management team and leading experts in the field of machine learning, autism and brain development, bridging the most advanced technological innovations with groundbreaking scientific research. The company is chaired by Brian Leedman, an experienced biotechnology entrepreneur and founder of ResApp Health, a digital diagnostic company recently acquired by Pfizer.
Key Technology and Applications
Neurobehavioral assays of brain function can reveal fundamental mechanisms underlying neuropsychiatric conditions, but typically require centrally located equipment in a laboratory test facility. Consequently, these tests are often unpleasant for participants, as they often require instruments attached to their face and cannot be used at scale in daily clinical practice, particularly with paediatric patients.
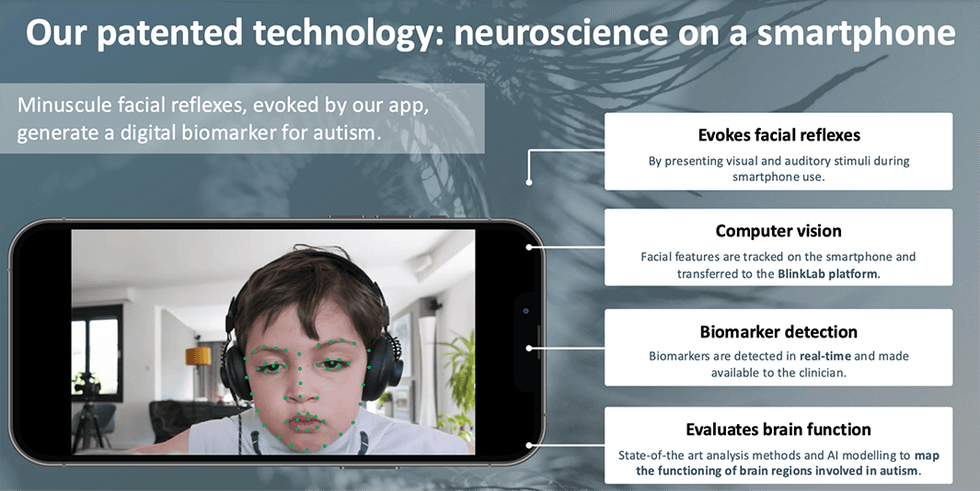
BlinkLab has developed a smartphone-based software platform, known as ‘BlinkLab Test’, to perform neurobehavioural testing that is free from facial instruments or other fixed location equipment.
This AI-based platform is designed to be used at home or in similarly comfortable environments, either independently or with the assistance of a caregiver, while following instructions from the smartphone application. The tests include, but are not limited to, eyeblink conditioning (EBC), which is a form of sensory-motor associative learning, prepulse inhibition of the acoustic startle response (PPI), which measures the ability to filter out irrelevant information through sensorimotor gating, startle habituation, which measures the ability for the intrinsic damping of repetitive stimuli and sensory adaptation, and habituation of the eye blink response, which serve as biomarkers for neurological and psychiatric disorders.
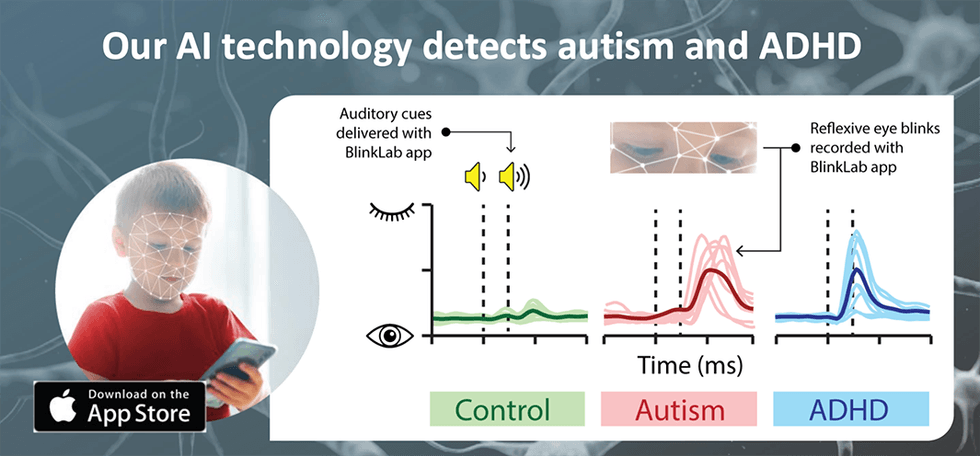
The BlinkLab Test App combines a smartphone’s ability to deliver stimuli and acquire data using computer vision with a secure cloud-based portal for data storage and analysis. In the tests, each audio and/or visual stimulus is presented with millisecond-precise control over parameters such as timing, amplitude and frequency. To maintain participant attention, an entertaining movie of choice is shown with normalised audio levels. Participants’ responses are measured by the smartphone’s camera and microphone, and are processed in real time using state-of-the art computer vision techniques. Response data is then fully anonymised, and transferred securely to the analysis portal. There, BlinkLab’s in-house AI/ML algorithms then perform clustering and statistical analysis to identify the prediction value of the experiment in the particular data set.
BlinkLab Test was initially developed as a prescription diagnostic aid to healthcare professionals (HCP) considering the diagnosis of ASD in patients 18 months through 72 months of age that are at risk for developmental delay. In collaboration with Princeton University in the United States and Erasmus Medical Center in the Netherlands, the company has conducted several trials using BlinkLab Test as an early assessment tool for autism. Autism represents a global challenge, with 1 in 36 children in the U.S. having autism, up from the previous rate of 1 in 44. With no early tests currently available to detect the condition, many children are diagnosed with the condition as late as the age of five.
Blinklab’s mobile app can aid in early detection, facilitating diagnoses as early as two years of age and resulting in earlier personalised interventions and monitoring. The testing process is also far more comfortable than traditional means of diagnoses, as the child can watch their favourite movie or cartoon on the phone, and the app will record their reactions, providing key information on the functioning of the brain.
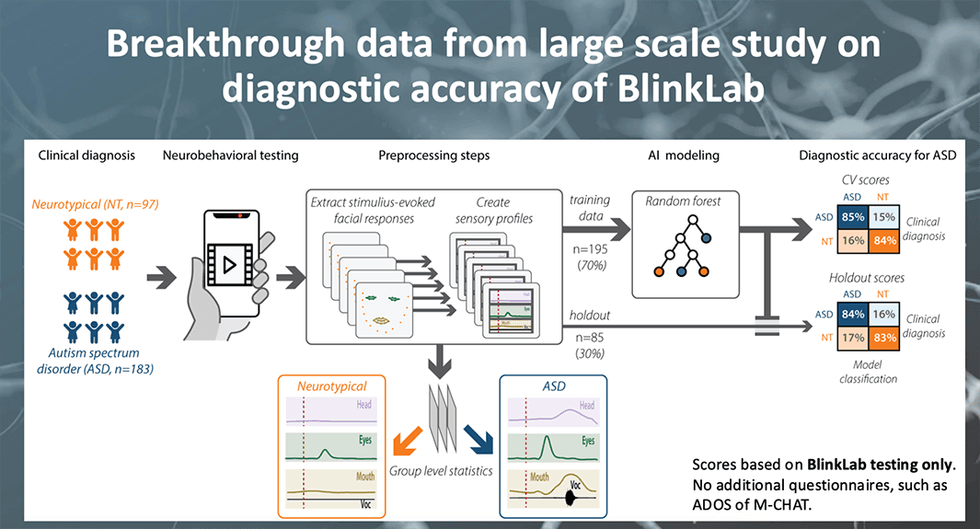
BlinkLab will be subject to regulatory oversight as a medical device and must clear clinical studies. Previous clinical trials completed by Blinklab have shown impressive indicators of success, achieving sensitivity levels of 85 percent and specificity levels of 84 percent. The company notes that these trials are very similar to those that are required by the United States Food and Drug Administration (FDA) for approval and have shown much higher levels of accuracy compared to currently approved products.
In order for the BlinkLab Test technology to be used as a clinical aid in the diagnosis of ASD, BlinkLab will need to complete a pivotal registrational study, and subsequently apply for FDA registration and reimbursement for the tests. The registrational study intends to recruit up to 500 subjects. Enrolment for this study is expected to begin in 2024, with study completion expected by mid-2025. The potential to participate in a disruptive and scalable AI-powered technology close to regulatory approval should attract attention from big medical technology companies.
Research and clinical studies
BlinkLab engages and partners with research and medical institutions across the globe to further test and develop its technology.
In May 2024, BlinkLab initiated a clinical study in partnership with US-based Turning Pointe Autism Foundation to enroll up to one hundred children previously diagnosed with autism and one hundred children without an autism diagnosis. The data obtained during this collaboration will be used to finalise the data collection and processing algorithms and AI/ML models ahead of the FDA registrational study.
The company is also participating in a clinical study of patients with spinocerebellar ataxias, conducted by Columbia University, New York, to study the effect of aerobic physical exercise on neuroplasticity in adults with spinocerebellar ataxias (SCA).
To further improve and accelerate the diagnostic evaluation of ADHD, BlinkLab forged a major research and clinical partnership with Mental Care Group (MCG) in The Netherlands, the fifth largest outpatient mental health care provider in Europe.
To validate BlinkLab’s platform for the assessment of functional neurological disorder (FND), the company has partnered with Bates College in Maine for a clinical study that aims to characterise the behavioural time course of Pavlovian eyeblink conditioning and acoustic startle habituation. It will validate the BlinkLab smartphone test for use as a remote neurobehavioral testing and diagnostic tool for FND.
At Erasmus University Medical Center, BlinkLab’s smartphone-based remote assessment, including eyeblink conditioning and prepulse inhibition of the acoustic startle reflex, is being used, among other tools, in a clinical study to set-up an overarching at-home testing lab, named the Digital Dementia Lab, to identify, develop and test a variety of digital biomarkers
measuring clinically relevant behaviour for improving early accurate diagnosis of dementia.
BlinkLab is also working with Monash University in Australia to evaluate BlinkLab as a medical device for monitoring the therapeutic effects of ketamine on cognitive processes whereby sensory information is converted into decision making. Results from this study can help facilitate cognitive behavioural therapy outcomes in patients with psychiatric conditions such as depression, schizophrenia, epilepsy, and post-traumatic stress disorder.
BlinkLab also recently signed a partnership for more clinical trialling with INTER-PSY, a large centre in the Netherlands that specialises in autism, offering assistance with diagnostics and treatments. This study also mirrors the study design of the Company’s developing FDA regulatory trial, which will be needed for future approval of BlinkLab Test as an approved diagnostic tool in the United States.
Management Team
BlinkLab is led by an experienced management team and directors with a proven track record in building companies and vast knowledge in digital healthcare, computer vision, AI and machine learning. The company’s chairman, Brian Leedman, is an experienced biotechnology entrepreneur and founder of ResApp Health, a digital diagnostic company for respiratory conditions, which was recently acquired by Pfizer for $179 million before reaching FDA approval for their main diagnostic product.
Dr. Henk-Jan Boele – Founder and Chief Executive Officer
Henk-Jan Boele is an assistant professor of neuroscience at the Medical Center of Erasmus University and a researcher at Princeton University. He obtained his PhD from Erasmus University in 2014. Boele has always been pushing scientific and methodological boundaries, and received numerous government and industry grants in the field of neuroscience.
Peter Boele – Founder and Chief Technology Officer
Peter Boele holds a bachelor’s degree in history and philosophy from Leiden University. He has over 20 years of experience in software development and has worked with Erasmus University, Leaseweb, Kaboom Informatics and Insocial.
Dr. Anton Uvarov – Founder and Chief Operational Officer
Anton Uvarov holds a Ph.D. from the University of Manitoba and an MBA from the Haskayne School of Business. He has rich experience in bio-technology investments with a particular focus on neuroscience and has successfully led several IPOs. He started his career as a biotechnology analyst with Citigroup, US.
Dr. Bas Koekkoek – Founder and Chief Scientific Officer
Bas Koekkoek is an assistant professor at Erasmus Medical Center. Koekkoek has been working at the Department of Neuroscience mainly in the role of rapid prototype of new technology and techniques for neuroscience. He has numerous publications in the area of brain development including Nature and Science journals.
Professor Sam Wang – Founder and Chair of Advisory Board
Sam Wang holds a PhD from Stanford University. He is a professor of neuroscience at Princeton University, has published over 100 articles on the brain in leading scientific journals and has received numerous awards. He gives public lectures on a regular basis and has been featured in The New York Times, The Wall Street Journal, NPR, and the Fox News Channel.
Professor Chris de Zeeuw – Founder and Scientific Advisor
Chris de Zeeuw is chairman of the Department of Neuroscience at Erasmus MC in Rotterdam and vice-director at the Netherlands Institute for Neuroscience in Amsterdam. De Zeeuw has received over 100 grants, including the Pioneer Award from ZonMw and the ERC advanced grant. In 2006, he received the Beatrix Award for Brain Research from Her Majesty the Queen; in 2014, he became an elected member of the Dutch Academy of Arts & Science; and in 2018, he received the international Casella Prize for Physiology.
Credit: Source link