After getting the green light from the FDA, Elon Musk’s Neuralink is ready to begin testing its controversial brain chip implants in paralyzed human volunteers. The company announced in an official blog post that it is now recruiting participants for a clinical trial to evaluate the safety and functionality of its technology.
“The PRIME Study is being conducted under the investigational device exemption (IDE) awarded by the FDA in May 2023 and represents an important step in our mission to create a generalized brain interface to restore autonomy to those with unmet medical needs,” the company wrote. “The initial goal of our BCI is to grant people the ability to control a computer cursor or keyboard using their thoughts alone.”
To achieve its goal, tiny flexible threads with electrodes would be surgically embedded in the region of the brain that controls movement.
“During the study, the R1 Robot will be used to surgically place the N1 Implant in a region of the brain that controls movement intention,” the company said. “Participants will be asked to use the N1 Implant and N1 User App to control a computer and provide feedback about the system.”
Once implanted, Neuralink claims its device could potentially read a user’s intended hand and finger movements and translate them into commands to control external devices.
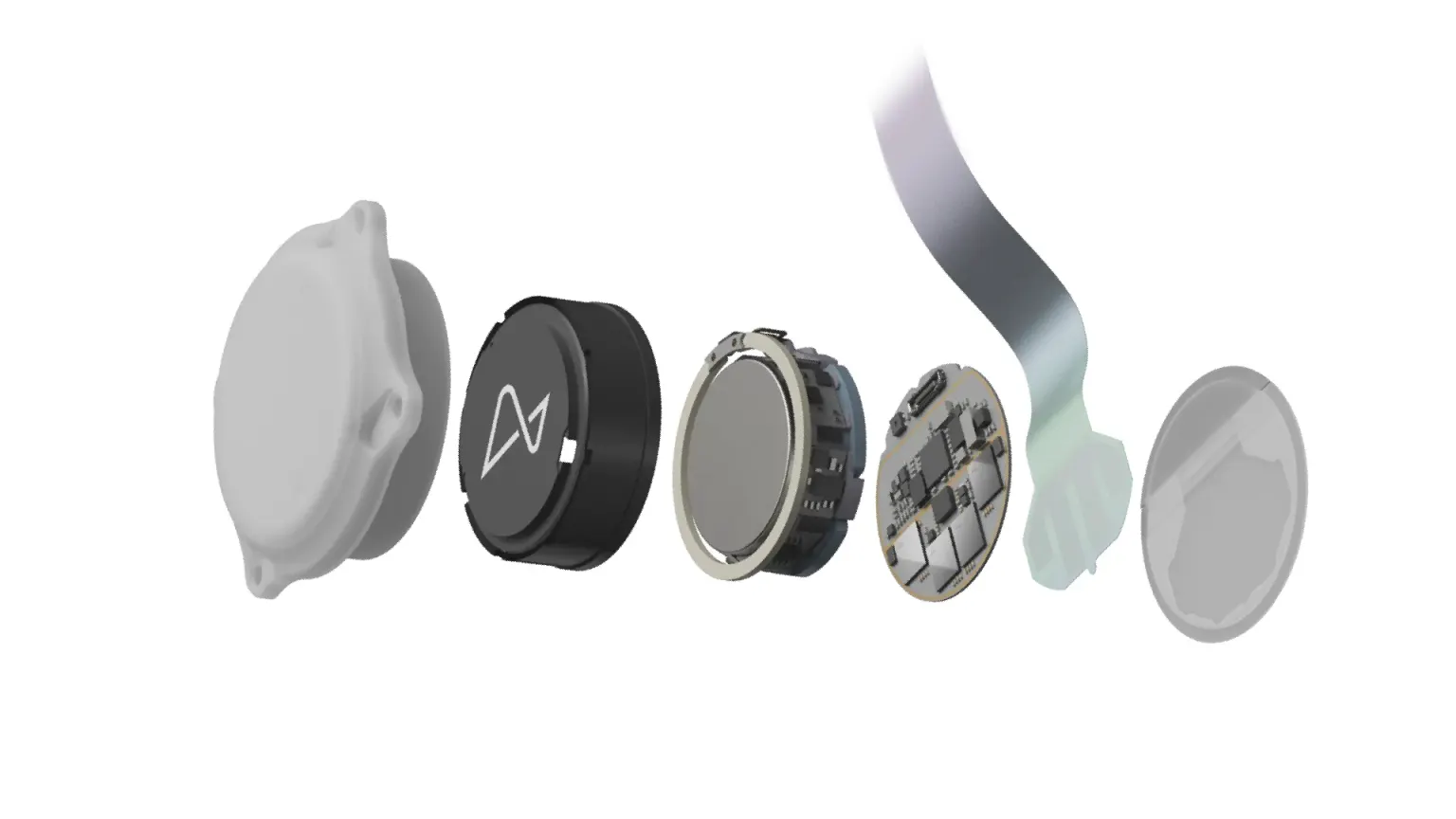
The N1 Implant is a chip that records neural activity. It measures around 8mm in diameter, but each of its 1,024 electrodes is thinner than a human hair, according to the company.
This highly invasive approach is not without risks, but other researchers have had recent breakthroughs in restoring mobility to paralyzed patients using less intrusive methods. As previously reported by Decrypt, quadriplegic Keith Thomas regained the ability to move his arm simply by thinking about it after scientists at Northwell Health implanted sensors on the surface of his brain.
If proven safe and effective, Neuralink’s brain chips could offer new independence to those suffering from paralysis due to spinal cord injuries or conditions like ALS. However, the technology also raises concerns about the ethics of implants that can literally read people’s thoughts.
Neuralink’s announcement follows a controversial history of animal testing. As detailed in a Reuters report, the company has been accused of botched experiments that resulted in over 1500 animal deaths.
Those willing to test the device will receive compensation for “ study-related costs” during the course of the six years that the study will last.
The experiment, called “The PRIME Study” (short for Precise Robotically Implanted Brain-Computer Interface), has been approved to start at a single, undisclosed hospital site for now. But Neuralink has grand ambitions for its tech, recently raising $280 million in funding, as reported previously by Decrypt.
Neuralink proposes a future where AI-powered, thought-reading chips are commonplace and augment human capabilities.
Still, many regulatory and ethical hurdles remain before tech billionaire Elon Musk’s vision becomes reality. While advances like Neuralink’s hold promise for patients with limited mobility, the line between treatment and human enhancement could become blurred.
Stay on top of crypto news, get daily updates in your inbox.
Credit: Source link